
Lauren Lawson, University of Toronto
Every fall, Canadians patiently wait for the turning of the trees and the crunch of leaves. In winter, we hear a different sort of crunch — the crunch of road salts.
Road salts are used to remove ice from surfaces like roads, sidewalks and parking lots. When people talk about road salts, they are often concerned with what salt may be doing to their vehicles, dog’s paws or winter boots.
There are also some environmental concerns, as road salt ultimately makes its way into our soils, local lakes and rivers. Salty water flows into our soils and local water bodies through surface runoff and stormwater pipes, and eventually makes its way into groundwater. This furthers the long-term storage of salt in the environment and further impacts freshwater aquatic life, government infrastructure and drinking water.
These concerns are usually voiced during the winter, when we actively see salt trucks and piles of salt on our drives or walks to work and school. While some of our worries disappear as the warm spring weather comes, my research shows the effects of extensive road salting on the environment last year round.
No longer a winter-only issue
My research with Donald Jackson at the University of Toronto showed elevated chloride concentrations — which are highly correlated with road salt — can now be found throughout the year in freshwater systems in the Greater Toronto Area. The impacts of road salt are not commonly studied in the summertime. However, understanding how it may be impacting the environment in the salt “low season” is important for understanding the gravity of the situation.
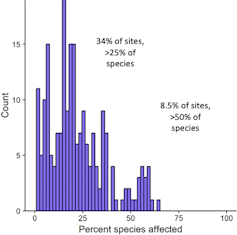
Our study found that during the summertime, which is also the low season for chloride, chloride concentrations exceeded established Canadian federal guidelines for protection of aquatic life.
At some of the sites monitored, we found that over 50 per cent of the aquatic biological communities can be considered to be stressed by chloride based on these guidelines, which were based on toxicity tests to aquatic organisms.
This means that summertime is now a time of likely chloride stress, higher water temperatures and early life stages of aquatic organisms (like eggs and larvae) which may be more sensitive to stress. These factors combined put aquatic species at elevated risk.
Why should we be concerned?
Road salt poses a risk for freshwater aquatic species, which rely on low salt levels. Freshwater species have specific biological adaptations to low salt levels, unlike their ocean counterparts which have different types of adaptations.
Studies show that increased chloride concentrations, associated with salt, can lead to disruptions in food webs, as sensitive species are stressed at high concentrations. For an aquatic organism, salt stress can lead to the diversion of energy to maintain basic functioning, which means less energy is directed to growth and reproduction.

High salt concentrations have been found to lead to decreases in egg mass for aquatic organisms, and decreases in growth rate. This essentially means sensitive species may eventually be “filtered out” of food webs, leading to declines in biodiversity.
Road salting leads to high concentrations of chloride and sodium in local waters. Increased chloride in drinking water supplies can lead to more rapid corrosion of drinking water infrastructure, such as private and municipal wells and pipes. This decreases the safety of drinking water. Increasing sodium concentrations is also concerning for those with hypertension.
To top it off, salting can lead to faster rates of corrosion of bridges and roads, putting road infrastructure at risk as well.
Lacking: efforts to reduce road salting
De-icing salts were first used in the 1940s in North America, and as its use exponentially increased with urbanization and road expansion, sodium chloride became the most popular. With increased understanding of risks to the environment and human health over time, efforts to reduce road salt use include using alternatives such as beet juice.
However, these alternatives can be expensive and can come with their own pitfalls, like introduction of more nutrients into aquatic systems. Understanding how much salt needs to be applied, and when, is a crucial part of salt and ice management. Additionally, shifts in perspective of ice safety can be made. In some regions for example, snow tires are required for vehicles while people use chains, boot spikes and other personal traction devices.
At a recent salt summit, held by the Lake George Association in New York, a speaker adequately described our current relationship with salt as “oversalting comes from a place of love, concern and want of safety,” because icy conditions are considered unsafe.
However, short-term prospects of ice safety blind us to the love, concern and want of long-term safety of our drinking water supplies and environmental integrity.
Mitigating our winter road salt addiction
We need to first recognize the year-round impacts our winter choices can have, and then take action to reduce the impacts. We can share the impacts of road salt and the individual actions we can take, such as understanding how much salt needs to be put down on our private properties, adjusting our expectations of winter roads and using snow tires and boot spikes to provide an added layer of safety.

At a larger scale, mandatory certifications for salt application can provide training for snow removal companies, and have substantial incentives if designed properly. For example, the New Hampshire Green SnowPro Certification provides limited liability relief for snow removal contractors if they are certified.
This ensures snow management companies are protected and their training programs are recognized as safe. Other organizations, like the Smart About Salt Council, provide the opportunity for certifications, training and general knowledge on salting.
Unifying the snow removal industry and scientific researchers is necessary to understand the full impact of salts, as understanding where salt is applied and how much is used is an important component of environmental modelling. This unification can be casual, such as through interviews.
It can also be more formal such as through joint research or educational initiatives, like the Partners in Project Green resource development for industry to understand road salt impacts and resources for more information.
Road salt pollution is an issue which can be acted on immediately, rather than relying on technological advancements, as action can be taken at the individual, the federal and all levels in between. This action should be taken swiftly to ensure a less salty future for our freshwater streams, lakes and drinking water.
So this winter season, when you hear the crunch of your boots on road salt, know that, although the salts we use now may not be visible after winter, the effect they have on the environment and our drinking water is year round.![]()
Lauren Lawson, PhD Student, Department of Ecology and Evolutionary Biology, University of Toronto
This article is republished from The Conversation under a Creative Commons license. Read the original article.


